Kolekce 138+ Atom Economy
Kolekce 138+ Atom Economy. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… For the general chemical reaction: Atom economy and percentage yield are indicators of how efficient a chemical reaction is.
Tady Atom Economy And Reaction Mass Efficiency Springerlink
Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The atom economy can be calculated in either of two ways: If both can be sold then there is no waste product and the atom economy is greater.Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.
The atom economy of a reaction is the percentage of atomic mass of useful products in a. Atom economy can be written as: The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. However, if this can be avoided it is more desirable, as Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Reactants desired product + waste products.

Atom economy can be written as: A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy can also be adjusted if a pendant group is recoverable. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy is the second principle of green chemistry. Atom economy can be written as: For the general chemical reaction: For the general chemical reaction:

Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Inefficient, wasteful processes have low atom economies. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The atom economy can be calculated in either of two ways:. Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... Reactants desired product + waste products. It is important for sustainable development and for economic reasons to use. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… The atom economy of a reaction is the percentage of atomic mass of useful products in a. Other articles where atom economy is discussed: For the general chemical reaction: Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Atom economy and percentage yield are indicators of how efficient a chemical reaction is.

If both can be sold then there is no waste product and the atom economy is greater. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. It is important for sustainable development and for economic reasons to use. Reactants desired product + waste products. In other words, atom economy is a. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.

Other articles where atom economy is discussed: Of a reaction is a measure of the amount of starting materials that end up as useful products.. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material.

A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. For the general chemical reaction:

If both can be sold then there is no waste product and the atom economy is greater.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Inefficient, wasteful processes have low atom economies. The atom economy of a reaction is the percentage of atomic mass of useful products in a. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted;. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%.
Other articles where atom economy is discussed: The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… For the general chemical reaction: Of a reaction is a measure of the amount of starting materials that end up as useful products. Atom economy is the second principle of green chemistry.. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Atom economy can also be adjusted if a pendant group is recoverable. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. For the general chemical reaction: Percentage yield and atom economy are two other practical considerations when doing chemical reactions. The atom economy can be calculated in either of two ways: However, if this can be avoided it is more desirable, as In other words, atom economy is a. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Other articles where atom economy is discussed:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Reactants desired product + waste products. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers.

Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Reactants desired product + waste products. However, if this can be avoided it is more desirable, as They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. For the general chemical reaction: The atom economy can be calculated in either of two ways:

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Atom economy can be written as: Other articles where atom economy is discussed: % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Atom economy is the second principle of green chemistry. Atom economy can also be adjusted if a pendant group is recoverable. The atom economy can be calculated in either of two ways: Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… Inefficient, wasteful processes have low atom economies. If both can be sold then there is no waste product and the atom economy is greater. Atom economy can also be adjusted if a pendant group is recoverable.
Atom economy can also be adjusted if a pendant group is recoverable... Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… The atom economy can be calculated in either of two ways: In other words, atom economy is a. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.
The atom economy of a reaction is the percentage of atomic mass of useful products in a. Of a reaction is a measure of the amount of starting materials that end up as useful products. The atom economy of a reaction is the percentage of atomic mass of useful products in a. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Atom economy can also be adjusted if a pendant group is recoverable. For the general chemical reaction: Atom economy can also be adjusted if a pendant group is recoverable.

% atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%... For the general chemical reaction: Other articles where atom economy is discussed: The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. The atom economy of a reaction is the percentage of atomic mass of useful products in a. It is important for sustainable development and for economic reasons to use. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Inefficient, wasteful processes have low atom economies. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… For the general chemical reaction: The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Inefficient, wasteful processes have low atom economies.
However, if this can be avoided it is more desirable, as.. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Inefficient, wasteful processes have low atom economies. If both can be sold then there is no waste product and the atom economy is greater. Of a reaction is a measure of the amount of starting materials that end up as useful products. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Atom economy is the second principle of green chemistry. However, if this can be avoided it is more desirable, as It is important for sustainable development and for economic reasons to use... Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. It is important for sustainable development and for economic reasons to use. The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. For the general chemical reaction: The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Of a reaction is a measure of the amount of starting materials that end up as useful products. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers.. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.

Other articles where atom economy is discussed:. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. Reactants desired product + waste products. Atom economy can also be adjusted if a pendant group is recoverable. Atom economy can be written as: A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Other articles where atom economy is discussed: The atom economy of a reaction is the percentage of atomic mass of useful products in a. Atom economy is the second principle of green chemistry. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

% atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%.. The atom economy of a reaction is the percentage of atomic mass of useful products in a. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. However, if this can be avoided it is more desirable, as For the general chemical reaction: Atom economy can be written as: Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Of a reaction is a measure of the amount of starting materials that end up as useful products. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. If both can be sold then there is no waste product and the atom economy is greater... Atom economy can also be adjusted if a pendant group is recoverable.

Other articles where atom economy is discussed: The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Reactants desired product + waste products.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Of a reaction is a measure of the amount of starting materials that end up as useful products. The atom economy can be calculated in either of two ways: However, if this can be avoided it is more desirable, as It is important for sustainable development and for economic reasons to use. The atom economy of a reaction is the percentage of atomic mass of useful products in a. Other articles where atom economy is discussed:.. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield.

Atom economy is the second principle of green chemistry. Atom economy can also be adjusted if a pendant group is recoverable. Atom economy can be written as: Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. In other words, atom economy is a.. Atom economy can be written as:

Atom economy and percentage yield are indicators of how efficient a chemical reaction is. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. It is important for sustainable development and for economic reasons to use.. Of a reaction is a measure of the amount of starting materials that end up as useful products.

% atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%... However, if this can be avoided it is more desirable, as A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; If both can be sold then there is no waste product and the atom economy is greater. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. For the general chemical reaction: % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Atom economy can be written as: Reactants desired product + waste products. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Other articles where atom economy is discussed:. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
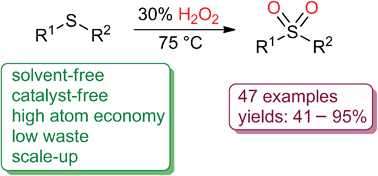
% atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. In other words, atom economy is a. If both can be sold then there is no waste product and the atom economy is greater. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield... The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.
Atom economy can also be adjusted if a pendant group is recoverable. Atom economy can also be adjusted if a pendant group is recoverable.. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield.

Other articles where atom economy is discussed: Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product.. For the general chemical reaction:

Atom economy is the second principle of green chemistry... Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Atom economy is the second principle of green chemistry. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. In other words, atom economy is a. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Atom economy can also be adjusted if a pendant group is recoverable.. However, if this can be avoided it is more desirable, as
The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product... Of a reaction is a measure of the amount of starting materials that end up as useful products. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Other articles where atom economy is discussed: Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was…

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The atom economy can be calculated in either of two ways: For the general chemical reaction: The atom economy of a reaction is the percentage of atomic mass of useful products in a. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. Atom economy is the second principle of green chemistry. Inefficient, wasteful processes have low atom economies.. The atom economy of a reaction is the percentage of atomic mass of useful products in a.

A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield.. For the general chemical reaction: Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Inefficient, wasteful processes have low atom economies. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *... % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%.

They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material... Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The atom economy of a reaction is the percentage of atomic mass of useful products in a. However, if this can be avoided it is more desirable, as.. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted;

Atom economy and percentage yield are indicators of how efficient a chemical reaction is... If both can be sold then there is no waste product and the atom economy is greater. The atom economy can be calculated in either of two ways: The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. For the general chemical reaction: Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The atom economy of a reaction is the percentage of atomic mass of useful products in a. Reactants desired product + waste products... Inefficient, wasteful processes have low atom economies.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. If both can be sold then there is no waste product and the atom economy is greater. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Reactants desired product + waste products. Other articles where atom economy is discussed: They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The atom economy of a reaction is the percentage of atomic mass of useful products in a. It is important for sustainable development and for economic reasons to use. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. If both can be sold then there is no waste product and the atom economy is greater.

For the general chemical reaction: The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. However, if this can be avoided it is more desirable, as

Reactants desired product + waste products. Atom economy can be written as: It is important for sustainable development and for economic reasons to use. The atom economy can be calculated in either of two ways: Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Other articles where atom economy is discussed: In other words, atom economy is a. The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.. Of a reaction is a measure of the amount of starting materials that end up as useful products. It is important for sustainable development and for economic reasons to use. For the general chemical reaction: Atom economy is the second principle of green chemistry. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Inefficient, wasteful processes have low atom economies. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted; Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers.. The atom economy can be calculated in either of two ways:

Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. For the general chemical reaction: Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… The atom economy of a reaction is the percentage of atomic mass of useful products in a. If both can be sold then there is no waste product and the atom economy is greater. Of these principles, "atom economy," originally suggested by american chemist barry trost in 1973, became a central concept among green chemistry researchers. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. It is important for sustainable development and for economic reasons to use. Atom economy can be written as: The atom economy can be calculated in either of two ways:

In other words, atom economy is a.. For the general chemical reaction: % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. If both can be sold then there is no waste product and the atom economy is greater. Inefficient, wasteful processes have low atom economies. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. In other words, atom economy is a. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Atom economy can also be adjusted if a pendant group is recoverable. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted;

Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. If both can be sold then there is no waste product and the atom economy is greater. Reactants desired product + waste products.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

Atom economy is the second principle of green chemistry. It is important for sustainable development and for economic reasons to use. Atom economy and percentage yield are indicators of how efficient a chemical reaction is. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.. Atom economy can be written as:

If both can be sold then there is no waste product and the atom economy is greater. It is important for sustainable development and for economic reasons to use.. A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted;

Other articles where atom economy is discussed: The ideal atom economy, known as 100% atom economy, for a chemical reaction is taken as the process where all reactant atoms are found in the desired product. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Reactants desired product + waste products. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was… If both can be sold then there is no waste product and the atom economy is greater. Atom economy can also be adjusted if a pendant group is recoverable.

The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. Of a reaction is a measure of the amount of starting materials that end up as useful products. However, if this can be avoided it is more desirable, as A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. Reactants desired product + waste products. In other words, atom economy is a. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.. The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *.

Of a reaction is a measure of the amount of starting materials that end up as useful products. The atom economy can be calculated in either of two ways: However, if this can be avoided it is more desirable, as

A) atom economy = 28/142 = 20% b) atom economy = 142/142 = 100% c) if only one product can be sold then the rest is wasted;. Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Efficient processes have high atom economies, and are important for sustainable development, as they use fewer natural resources and create less waste. Other articles where atom economy is discussed: The atom economy (atom utilisation) of a chemical reaction is a measure of the percentage of the starting materials that actually end up in useful products *. They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. However, if this can be avoided it is more desirable, as Atom economy and percentage yield are indicators of how efficient a chemical reaction is. The atom economy of a reaction is the percentage of atomic mass of useful products in a. Atom economy can also be adjusted if a pendant group is recoverable.. Percentage yield and atom economy are two other practical considerations when doing chemical reactions.

The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products.. Inefficient, wasteful processes have low atom economies. In other words, atom economy is a. However, if this can be avoided it is more desirable, as Of a reaction is a measure of the amount of starting materials that end up as useful products. Percentage yield and atom economy are two other practical considerations when doing chemical reactions. Atom economy can be written as:. A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield.

Mar 19, 2019 · the atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. Atom economy can be written as: They are both related to the amount of useful product generated in a reaction, compared to undesirable side products or unreacted starting material. In other words, atom economy is a. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. The atom economy of a reaction is the percentage of atomic mass of useful products in a. Reactants desired product + waste products.

Reactants desired product + waste products. . It is important for sustainable development and for economic reasons to use.

However, if this can be avoided it is more desirable, as Atom economy is the second principle of green chemistry. For the general chemical reaction: However, if this can be avoided it is more desirable, as If both can be sold then there is no waste product and the atom economy is greater. The atom economy can be calculated in either of two ways: A) atom economy = 100% b) the reversible reaction symbol ( ) suggests that the reaction is unlikely to have a very high yield. In other words, atom economy is a. Of a reaction is a measure of the amount of starting materials that end up as useful products. Reactants desired product + waste products. % atom economy = (molecular weight of desired product ÷ molecular weight of all reactants) × 100 atom economy can be poor even when chemical yield is near 100%. Atom economy was designed to overcome the limitations of the traditional concept of "yield," the amount of final products, which was…
The atom economy of a chemical reaction is a measure of the percentage of reactants that become useful products. The atom economy of a reaction is the percentage of atomic mass of useful products in a. In other words, atom economy is a.
